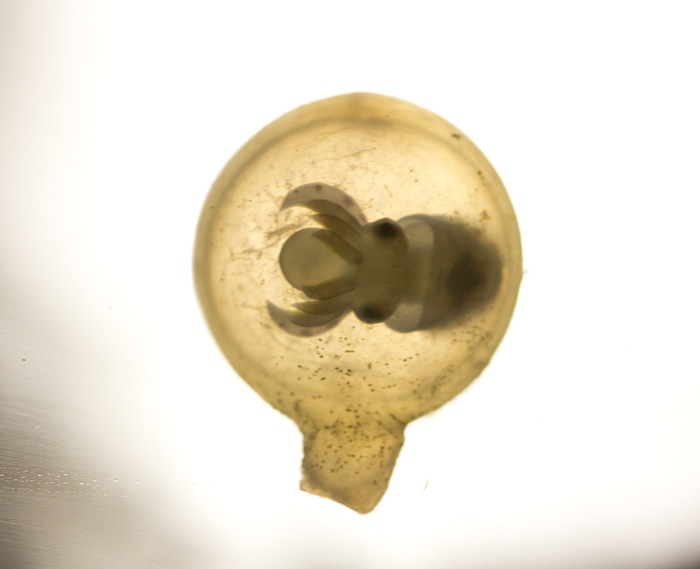
2011 Philippine Biodiversity Expedition – Part 1
Planning, Science and Surreality
From Reefs Magazine
by Richard Ross
Researchers from the California Academy of Sciences have been visiting an area called the Verde Island Passage off the coast of Batangas Province on Luzon Island, Philippines for almost 20 years. Research by scientists during that period suggested that this area is the “center of the center” of marine biodiversity, and perhaps home to more documented species than any other marine habitat on Earth; there can be more species of soft corals at just one dive site in this area than in all of the Caribbean. Thus it was only natural that when the Steinhart’s 212,000 gallon reef tank was designed, the Academy decided to represent the reefs of Luzon. Ever since, Steinhart biologists have traveled to this area in small groups with the objective of acquiring first hand knowledge of the environments they hope to recreate in San Francisco.
The 2011 Philippine Biodiversity Expedition, however, was a trip of a completely different magnitude: the largest expedition in the Academy’s history covering both land and sea. Consisting of a Shallow Water team, Deep Water team and a Terrestrial/Fresh Water team, the 2011 Philippine Biodiversity expedition, funded by by a generous gift from Margaret and Will Hearst, was the most comprehensive scientific survey effort ever conducted in the Philippines. Joining the expedition were over eighty scientists from the Academy, the University of the Philippines, De La Salle University, the Philippines National Museum and the Philippines Bureau of Fisheries and Aquatic Resources, as well as a team of Academy educators whose mission was to share the expedition’s findings with local community and conservation groups as the Expedition was happening.
This video by Bart Shepherd show some of the reefscapes we encountered on the 2011 Philippine Biodiversity Expedition
As part of the Shallow Water team Bart Shepherd, Matt Wandell and I surveyed and further documented the underwater sites that served as the inspiration for the Steinhart’s Philippine Coral Reef exhibit. We also responsibly collected coral, cephalopods and other invertebrates for captive propagation, research and display at our Golden Gate Park facility. As the only public aquarium permitted to collect stony corals in the Philippines, we were to obtain these unique species for study, captive culture research, distribution to other institutions as well for display at the aquarium.
Planning
On previous trips to the Philippines, Steinhart biologists had been given special permission to collect and export small numbers of small coral fragments, most of them collected as ‘found frags’. The 2011 Expedition would continue this tradition, albeit with some modifications. In order to reduce stress on the organisms, we planned to adopt Ken Nedimyer’s work with the Coral Restoration Foundation (http://www.coralrestoration.org/) to create a system for holding our coral fragments offshore until transport. We mocked up the system using materials that we could travel easily with, or that we could find in the field – silicone airline tubing, zip ties, dive weights and empty plastic water bottles as floats (after all, you can sadly find empty plastic water bottles on just about any beach in the world). The mock up went into our big reef tank for testing and was immediately dubbed ‘the coral clothesline’ by the aquariums docents.

In addition to our clothesline supplies, we packed everything else we could think that we might possibly need. Some highlights: six large, low style plastic tanks that could be weighted and sunk offshore for holding larger fish and other inverts, as well as smaller plastic tanks that could be hung from the Clothesline to hold small fish and other inverts. A backpack kit for harvesting jelly gonads (as removing the gonads doesn’t impact the jellies long term and the gonads ship better than adult jellies). Fiberglass window screen to make lids for impromptu holding containers, as well as the rubber bands to hold those lids on. Dozens of tubes of super glue and rolls of duct tape. LCD microscope just in case we needed to look at something close up. Sharpies for note taking. Scissors for cutting everything. Needle nose pliers for coral fragmenting. Plastic rulers for scale in photographs. Deli cups for transport, collection and shipping or animals. All this stuff and more went into one fish shipping box and filled every empty space in our luggage.
After flying all night to Manila, all this gear, along with some very tired biologists, hit the ground running at 5am, finding our checked items, finding our ride and driving 3 hours to Club Ocellaris, a world renowned SCUBA resort, which would be the base of operations for the shallow water team.
Science was everywhere

When we arrived at Club Ocellaris, we found it had already been completely taken over by the Expedition. Science was everywhere. Across the resort, any flat space had already become some sort of makeshift lab, with equipment and apparatus piled all over the place. Every electrical outlet had a computer, camera, light or batteries charging. Containers of every conceivable kind from plastic bags, to lidded jars, to 5 gallon buckets waited everywhere to be filled with hunks of science. While all of that was exciting, we really wanted to get in the water. Within an hour of arriving our Diving Safety Officer, Elliott Jessup, got us suited up and on a boat for our first dive of the trip – we saw sea snake, corals and fish galore. After our afternoon dive, we assembled our offshore holding about 50 meters offshore so we would be ready for whatever collecting we would do the next day. When we were done, we were treated to the most spectacular sunset I have seen in a long time. Not a bad way to start off the expedition.

The overall dive plan for the Steinhart Biologists was to dive and collect for 6 days, then drive the animals we had obtained back to Manila for ship out on our ‘dry day’ (to give our bodies a chance to off gas Nitrogen in our tissues from diving), drive right back to Club Ocellaris for another 6 days of diving and collecting, then back to Manila for packing and shipping then fly home the next day. The daily schedule of activities would be a grueling marathon, but we couldn’t wait to get started.
Life in the expedition
Every morning, we woke at around 6 am for coffee and Skype video calls to home and work where it was 2 pm the previous day. Breakfast (ummm, mango shakes) and our dive briefing started at 7 am. With up to five dive boats going out each day, coming to agreement on where we would be diving was no small act of coordination. After breakfast we would collect and test our NITROX tanks for the day, get our cameras and collecting gear ready, and assemble & check our dive gear and load it onto the boats. Then we would suit up and zoom out to a good place to get under the wanter.
On each dive, we not only collected animals, but also completed multiple steps designed to track each specimen – every coral fragment was photographed and assigned a number that provided information on when it was collected, from what dive site and depth it was harvested, as well as the name of the biologist who collected it. Each coral got at tag attached to it so we would, in theory, be able to ID it later. The tagging was a learning experience and morphed over time, so much so, that next year we will most likely use heat stamped numbered zip ties as tracking id’s, but attach those zip ties to the coral with 20 gauge coated wire the tips of which are sealed with a rubberized plastic dip because the wire will be easier to manipulate and create less waste than other methods we tried.

After the second dive, we would head back to land and offload our animals. From the dock, we would change our scuba equipment for snorkels, and then swim our new specimens to the off shore holding facility, often making multiple trips. Then we would eat ravenously, then turn around and repeat the same process for the afternoon dives.
We would finally climb out of the water at 6pm for dinner… unless we were doing a night dive. On night dive days, dinner and dry-off was often as late as 10 pm.

After dinner, there was more sciencing to be done. The spreadsheet detailing what we had collected needed to be brought up to date, the Coral ID software needed to be consulted to identify each SPS coral to species. Paperwork for permits for export, and shipment/arrival details needed to be initiated and updated. When that was done, we were often drafted to help the researchers on other teams process specimens they had collected, take pictures, be all around helpful, and tend to whatever animals we were keeping onshore. Sometimes we even had a moment to geek out with Philippine scientists, or have a drink of the local rum (which I still think also contained Formalin). We were lucky if we fell into bed by 11:30.
The second night
Our first night dive was something special. The moon was full and the dive site wascalled Dead Palm. We hit the water just after the sun set to swim over stands of Acropora of all different kinds and Turbinaria colonies as large as a car. It was an SPS lover’s dream dive. About halfway through the dive the particulate in the water started to gradually become noticeably thicker, and virtually at the same time the three of us looked at each other and yelled SPAWN through our regulators.
Many corals reproduce in coordinated mass orgies where they release millions of gametes into the water. None of us had ever seen a coral spawn in the wild, and it really is as cool as it looks in the documentaries. We traced the spawn to a huge thicket of Acropora nobilis, and watched in amazement as each egg/sperm bundle emerged from the branches and floated towards the surface where fertilization takes place. Within a few days the fertilized eggs change into a coral planula, coral larvae, which swim around (yes, swim!), until they find a suitable place to settle and develop into a mature coral.
Coral spawning is one of the new frontiers in captive coral reproduction, because collecting spawn instead of coral fragments can yield many more corals in captivity in an incredibly sustainable collection method. A group of public aquarists and coral scientists formed SECORE (SExual COral REproduction – http://www.secore.org/) and they have been holding workshops in the Caribbean for the last several years to perfect spawning, fertilization and settling procedures. Building on the success of the Caribbean workshops the Steinhart Aquarium hopes to hold a SECORE workshop in the Philippines in the next few years. The most important part of such a workshop is of course timing it with the coral spawn. There is not much information on the timing of Philippine coral spawns, and none of the previous trips to the area had ever come across one, so actually observing coral spawning in the Philippines is a good and necessary start to bringing SECORE to the area.
We, along with some of the other California Academy of Sciences researchers and a Philippine television crew, returned to Dead Palm the next night where the coral spawn was in full swing yet again. We were able to find a colony of Acropora willisae when it was beginning to release gametes and set up around the coral to both collect some of the spawn and to document the event. I’ll never forget filming Matt collecting gametes in a plastic bag while the television crew was filming me film him. We were able to collect several hundred sperm and egg bundles, and though completely unprepared for the labor intensive process of fertilization and settlement, were gave it a go.
My video of the coral spawn and the gamete collection
A surreal night
After years of having the privilege of diving around the world practicing no impact diving, after collecting for the trade practicing and teaching having as little impact as possible, and after planning to take ‘found frags’ when possible, watching a scientific survey on the move takes a bit of getting used to. The researchers were collecting everything – worms, urchins, fish, nudibranchs – and just about every dive on the Expedition yielded at least one animal that seemed to be undescribed by science. The animals were being collected and preserved for scientific description, genetic analysis and as a way to be comprehensive in the survey, and being in the midst of a full on scientific survey lead to the Steinhart biologists to try to take advantage of the situation, and alter our plan regarding what we would try to bring back to San Francisco for our living collection.
On the third evening of our diving, Dr. Healy Hamilton showed us some ghost pipefish, Solenostomus cyanopterus, and some pygmy seahorses, Hippocampus bargibanti, that had been collected that day. These animals were going to be sacrificed for their genetic material. I know some people have a visceral reaction to that idea, but as Dr. Gerald Allen once said during a MACNA talk “It’s a necessary part of science”. Of course when we saw the ghost pipefish, a species that we had always wanted to work with but hadn’t because of their dismal record of surviving collection and shipping through the trade, we immediately suggested that we try to keep them alive and that we try to ship them home and put them on display – if they didn’t make it, we would still have their genetic material available for science. Though we weren’t prepared for holding these kinds of animals, Bart, Matt and I had been trained in the ultimate McGyver proving ground – the reefkeeping hobby. We got to work setting up buckets aerated by scuba tanks, faux gargonian hitching posts for the seahorses made from zip ties, and prepared ourselves to do water changes as often as needed by slogging 5 gallon buckets up and down 2 flights of stairs.
Of course, the third night of diving was also the night we collected coral spawn, so while we were preparing to try to keep these amazing fish alive, we were also preparing to attempt to keep the coral spawn healthy and fertilized which included ‘stirring’ the gametes every hour or two. This led to the most surreal night of the trip. We had coral out on the clothesline, ghost pipefish in the offshore holding tanks, trays of coral eggs and sperm, and a bucket with two pygmy seahorses next to our beds. Throughout the night we kept waking up and tending to these animals – a strange, wonderful and exhausting time.
In the end, we were successful keeping the ghost pipefish alive, and getting them home to the aquarium in Golden Gate Park. Sometime in the night we noticed that the pygmy seahorses were no longer living, and we preserved them. The coral spawn failed to thrive, and it seems that we were simply too unprepared and understaffed to have succeeded in that labor intensive realm. We learned a lot and helped move science forward. Of course, we plan that for next year’s trip, we will be much more prepared for new surprises and opportunities.
In the next installment – coral collecting, octopus wrangling, shipping & packing for the trip home, and 300-500 new species discovered.
Special thanks to Bart Shepherd, Matt Wandell and Elizabeth Palomeque
Rearing the Flamboyant Cuttlefish – Not by Rich Ross
From Reefs Magazine
by Allison Petty
Photos by Christopher Paparo, Video by Richard Ross
The Holy Grail
As a professional aquarist, my career has presented me with the opportunity to work with a variety of remarkable marine life. Working with animals from sharks to mammals, and electric eels to reptiles has been very rewarding, but none compare to the experience of working with cephalopods. At Atlantis Marine World, I care for our two cephalopod exhibits, the giant pacific octopus and cuttlefish. They are two of the most popular exhibits at the aquarium. Their unique, almost alien-like appearance, combined with their ability to change color and shape in an instant, keeps visitors mesmerized in front of the exhibits all day. Sadly, the specimens kept in these two exhibits are not with us for long. All cephalopods have a very short life span, some lasting less than a year. They hatch, grow quickly, and die shortly after reproduction. Fortunately though, this short life means they reach sexual maturity in a reasonable amount of time, making captive breeding of many cephalopod species possible. Typically, we keep Sepia officinalis or Sepia pharaonis, and I have been fortunate enough to raise both species. Recently, however, a twist of fate has afforded me the opportunity of a lifetime.
This journey started almost a year ago when a marine life wholesaler in California called to tell us that he had some Metasepia pfefferi (flamboyant cuttlefish) coming in and asked if we were interested. Since it is considered the holy grail of cephalopods and probably the coolest animal on the planet, what could we say?
The M. pfefferi was being sent next day via FedEx, which meant there was little time to prepare. Swinging into high gear we quickly set up a home for it, which ended up being a 24-gallon Via-Aqua tank with a shallow bed of live sand. The cuttlefish arrived the very next day. We acclimated it to its new home, and I immediately fell in love. Since I have never taken care of a flamboyant cuttlefish before, I contacted Richard Ross, the “cephalopod guru”, to ask for any useful information. He told me that it is common for flamboyant cuttlefish to mate before being collected. He explained that they prefer to lay their eggs under ledges and he recommended adding coconut shell halves in the tank, just in case by some miracle we received a gravid female. It seemed like a long shot that this cuttlefish could have reached sexual maturity and mated already, as it was only 2.5 inches in length.
After giving the cuttlefish some time to settle in, we offered it a live shore shrimp (Palaemonetes pugio), which it immediately stalked and devoured. The flamboyant is like no other cuttlefish I’ve encountered before. Most cuttlefish are masters of camouflage, having the ability to blend in quite well with their surroundings by changing the texture as well as the color of their skin quickly. Flamboyant cuttlefish share this ability to blend, but can also take their appearance to the other extreme with their stunning coloration. When they feel threatened they show a remarkable rippling display of colors down their body from bright yellows and whites, to bold purples and reds, making them stand out vibrantly. This show of colors is also a way to broadcast to potential predators that it is poisonous. It is said to be as lethal as a blue-ringed octopus, making the flamboyant cuttlefish the most toxic of the cuttlefish species. Another odd behavior of the flamboyant is that unlike other cuttlefish that are usually shy and spook easily, flamboyant cuttlefish are courageous. They will stand their ground instead of jetting off into the background of their tank. These behaviors make them very intriguing and guaranteed to hypnotize anyone. Needless to say, none of us got much work done for the rest of the day.
As the flamboyant was settling in and getting comfortable in its home, I added it into my routine of daily feedings and water changes. Being that Atlantis Marine World is located on a tidal river, it is very convenient to get endless amounts of shore shrimp and killifish. These shrimp and killifish are enriched with Cyclops and salt-water mysis before they are fed out. Using live food helped maintain good water quality since any uneaten food would be alive and not foul the water. However, being that it was a new system and cycling, I did a 15% water change, 3 times a week in order to keep the ammonia, nitrates and nitrites as close to zero as possible. I kept the salinity around 33 ppt, the pH between 7.8 and 8.0 and the temperature close to 73 degrees Fahrenheit. This combination seems to keep the flamboyant happy and healthy.
After about 3 weeks of giving this flamboyant a lot of special attention the unthinkable happened: she laid eggs! The morning of July 4th was a memorable one to say the least. On my morning rounds, I stopped to say good morning to her and to my surprise there were about 20 perfect white eggs in one of the coconut halves. Ecstatic beyond belief, I needed to find someone to share my excitement and that someone happened to be Senior Aquarist, Chris Paparo. He told me not to get too worked up because he thought there was a high chance that they were infertile. However, I had a strong feeling otherwise and was excited to watch them develop.
With the exception of marine mammals and a few other taxa (damsels, cardinals, crustaceans, etc.), maternal instincts are lacking in the marine world. Most marine organisms release egg and sperm into the water, and hope for the best. The flamboyant cuttlefish is one of those exceptions. Most of the day she spent tending to the eggs, keeping them clear of detritus and other fouling agents, and guarding them from possible predation. Even though she was alone in the tank and there was no predation threat, she would still “pace” back and forth in front of the shells using her tentacles and two leg-like appendages that looked as if they were molded from the bottom of her mantle. Instead of swimming, flamboyant cuttlefish spend most of their time literally walking around on the substrate. This benthic behavior is due to their smaller than normal cuttlebone. All cuttlefish have a cuttlebone, which is made up of calcium carbonate. It is divided into chambers and depending on the buoyancy that a cuttlefish needs, it can either empty or fill these chambers with gas. Since the flamboyant cuttlefish has a small cuttlebone, they have a harder time with their buoyancy and cannot swim for long periods of time without sinking.
On August 10th, approximately a month after the first egg was laid the unimaginable happened and possibly the most important day of my career had come. While giving out her first feed of the day, I noticed the most beautiful, tiny baby cuttlefish hanging out on the wall of her tank. Not believing my eyes, standing there in shock and awe, fellow Aquarist Todd Gardner rounded the corner and asked what I was looking at. As I showed him the 1 cm long carbon copy of the adult flamboyant we stared in silence together, then celebrated for about 5 minutes before starting to think about setting up a tank for Junior.
Here at Atlantis Marine World, we believe in keeping things simple. So for Junior’s tank we used a 10-gallon tank with a hang on the back Aqua Clear mini filter and some live aragonite as substrate. After the new system was running and ready for its first occupant, I carefully scooped up the tiny baby in a deli cup and gently transferred it into its new home. Now for the hard part, what to feed this little guy? After doing some research, I found that newly hatched mysid shrimp were needed to feed Junior. I located a company in Florida, Marinco Bioassay Laboratory, which cultures mysids. After making a call, I ordered the smallest possible mysids they could ship me, which were 7 days old, and hoped it would be suitable. To my relief they were and it didn’t take long for Junior to track them down and consume them. Keeping the water parameters of the tank as close as possible to its mothers was easy enough; it was keeping the right amount of food in the tank that was more difficult. Too few mysids made catching one more difficult, but too many would stress Junior.
http://vimeo.com/21888992
Over the next two months ten babies hatched. Unfortunately, two of these hatched prematurely. The two preemies had buoyancy issues and one still had a yolk sac. Needless to say, they did not survive. Keeping all eight hatchlings in the same tank worked at first, but started to become an issue when it came to feeding. Since there was a 2-month difference from the oldest to the youngest, the oldest seemed to be over powering the little ones and eating a majority of the food. At 2 months, the oldest was big enough to eat something more substantial than mysids, so I searched through the shore shrimp tank to find the smallest shore shrimp possible. At about a quarter of an inch long, I broke off the rostrum of the grass shrimp and dropped it in the tank in front of the oldest baby. To my delight he ate it right up. The feeding process started to get tedious and time consuming. It was a real challenge to make sure that all of the babies were getting enough food, so more tanks needed to be set up.
The 10-gallon set up was working just fine, so I set up two more 10-gallon tanks and another 24-gallon Via-Aqua. I size sorted the babies and split them between the four tanks. This seemed to work out well, especially when it came to feeding. Being able to see how much they were eating and weaning them from mysids to grass shrimp was easier and less stressful. Although feedings were simpler, I increased my maintenance workload by three-fold. I was still water changing their mothers’ tank 3 times a week, and now having to do the same for the four baby tanks was repetitive yet necessary to keep up with the proper parameters for these guys to grow and be healthy.
All the work I’ve been putting in with these guys was challenging and monotonous at times but it was beyond worth it. When the oldest babies reached ages of about 4-5 months, they were big enough to be displayed. Getting the “o.k.” to redo the existing cuttlefish exhibit, I replaced all the substrate and décor and revamped the overflow so the smaller cuttlefish would not get stuck to it. Once finished, I moved 6 flamboyant cuttlefish to the 500 gallon half circle exhibit. They got along for the most part. Surprisingly it was the smallest one that caused some trouble. He would get up in the others’ faces, follow them around, and threaten them. Basically it was like he had Napoleon complex and was trying to prove himself. This lasted for about 2 weeks before they all settled down, made peace with each other and made excellent display animals.
Meanwhile, back behind the scenes, their mother was still going strong. She continued to lay hundreds of eggs and took great care of them. There were no more fertile eggs by this point, but she still acted as if there were by guarding and cleaning them. She continued to eat very well until mid-January. Her eye sight started to go and she was missing her food. Like all Cephalopods when it is their time to go it is very sad to watch as they slowly perish. By my calculations she was at least 14 months old, and knowing cephalopods are short lived, I figured she had lived a long, fruitful, and what I hope was a happy life. January 27th was a sad day for me as my first and yet very successful Flamboyant cuttlefish had passed. With all that I have learned from her, I hope I get the chance to repeat this process. All I can do now is wait and see if her legacy will live on with the hope that the courtship I am seeing with the new generation will be equally bountiful.
http://vimeo.com/26608076
UPDATE: As of this writing, Allison’s hopes for the second generation have been realized as evidenced by the photos and video below. Several captive bred specimens have now been reared and sent to other aquariums for further study. Congratulations on yet another stunning achievement!
Skeptical Reefkeeping part 4 –What does that even mean?

Display, Husbandry and Breeding of Dwarf Cuttle, Sepia bandensis, at the California Academy of Sciences
From Drum and Croaker 2010 Issue
Display, Husbandry and Breeding of Dwarf Cuttle, Sepia bandensis, at the California Academy of Sciences
Richard Ross, Aquatic Biologist, Steinhart Aquarium, California Academy of Sciences,
55 Music Concourse Drive, Golden Gate Park, San Francisco CA, 94118 USA
Cuttles seem to always be on the want list for public aquarium displays; however, the species generally available, Sepia officinalis and Sepia pharaonis, require large exhibits which can be a major commitment in both husbandry and cost. It can also be difficult to justify the commitment of show space and resources to such short-lived animals (the life span of most cuttles is typically between 1 and 2 years). However, the dwarf cuttle, Sepia bandensis, is a species that can be housed in a much smaller exhibit than its larger cousins making it an attractive first step into public cuttle displays. Sepia bandensis perform all the exciting and interesting behaviors that make cuttles popular, and can even mate and lay eggs while on display. Even better, S. bandensis are not prone to damaging themselves by jetting into the sides of aquaria. At the California Academy of Sciences, we have been displaying S. bandensis for almost a year, the animals have successfully bred on display and we are well into raising our second generation.

Procurement of animals, hatching of wild eggs, and housing of hatchlings
Sepia bandensis eggs are commonly available twice a year, around March and October, from marine wholesalers. In April 2009, the Aquarium received 3 egg clusters of approximately 20 eggs each, from Quality Marine in Los Angeles. The eggs were housed in a 5 gallon critter keeper sitting in a weir box of one of our back of house coral grow out systems. Water quality was coral compatible; temp 26C (78F), salinity 33-35 ppt, pH 8-8.4, calcium 380-400 ppm, alkalinity 7-9 dKH, ammonia 0, nitrate 0-10 ppm (NO3), PO4 0.05 ppm or below.
Water was supplied to the critter keeper by a Maxi Jet 1200 power head in the sump of the system, with the flow rate controlled by a ball valve. The water gravity drained through the slotted lid into the weir box. There was enough flow to gently keep the eggs ‘swaying’ and there were several cupfuls of fine sand on the bottom. By the end of April roughly 50 of the eggs had hatched and the hatchlings were kept as a group in the critter keeper. At this point the water flow was increased in order to keep any food items moving because cuttles hunt moving prey. Traditionally, it seems that hatchling cuttles have been kept in low flow environments, but it seems they do very well in higher flow captive environments as well. Since the hatchlings spend most of their time on the substrate, there was never had a problem with animals getting trapped against the lid as the water overflowed back into the system weir box.

Feeding hatchlings
The biggest challenge raising S. bandensis from eggs is feeding the hatchlings. The challenge is twofold – appropriately sized food and getting enough of it. As the hatchlings grow, the size of the prey item offered needs to increase, and getting enough of any appropriate prey items can be costly. The Aquarium was able to supplement purchased food items with locally caught amphipods, locally caught freshwater mysids, and when the cuttles were larger, locally caught shore crabs of various species. It is also possible to wean juvenile cuttles onto thawed frozen foods, but this should be supplementary to live foods – more on this later.
Two quick notes on feeding hatchling S. bandensis: 1) Sometimes, hatchlings don’t appear to eat for the first week or so after hatching. It may be the case that they are actually not eating and may still be feeding on the remnants of their yolk sack; it may be that they are eating after lights out; or they may be eating small amphipods or copepods already present in the aquarium. In any event, they seem to come out of it and begin eating voraciously. 2) Enough people have anecdotally tried and failed to raise hatchling S. bandensis on enriched Artemia that I don’t think anyone need to try it again (although a study might be informative).
For the first few weeks after hatching, the hatchlings were fed twice a day with live mysids from Aquatic Biosystems and Aquatic Indictors. Mysid shrimp were gut loaded with newly hatched Artemia . Live mysids seem to be a perfect food because they are easily caught by the hatchling cuttles. After several weeks, amphipods were introduced into the diet. There seems to be a learning curve to the hunting ability of hatchling S. bandensis; amphipods are strong and when introduced too early in S. bandensis development, they are able to easily escape from the hungry cuttles possibly causing damage.
Around week 4, locally collected fresh water mysids were introduced into the diet, which the cuttles were able to catch and consume before the shock of being placed into saltwater stunned them into no longer moving.
Around week 6 we began to introduce thawed and rinsed frozen Piscine Energetics (PE) mysids into the diet. Initially, these were placed into the aquarium along with live mysids. Because of the decent flow rate, the cuttles would strike at the PE mysids as they was blown around in the water column. Within a week, one of the daily feedings became solely thawed PE mysids.
Around week 8, our hatchlings were between 1.2 cm (½ inch) and 2.5 cm (1 inch) in mantle length, and larger foods became necessary both from a nutritional and cost perspective. Fresh water ghost shrimp were available from a local wholesaler, however, keeping these alive long term became challenging. Marine ‘janitors’, Palamontes vulgaris, from http://livebrineshrimp.com/ were purchased and easily housed long term in a 20 gallon tank with an air driven sponge filter. These shrimp were approximately the same length as the cuttles and were readily consumed.
Feeding adolescents and adults
Once the S. bandensis were larger than 2.5 cm (1 inch long), saltwater grass shrimp, Cragnon spp. were purchased from a local bait shop and introduced into the diet. The Cragnon were kept in an auxiliary aquarium on a coldwater system kept at 11C (52F). Purchases of Cragnon include shrimp of different sizes, so it is easy to pick out the best size for the S. bandensis – even though at this size the cuttles can easily take prey larger than themselves. From time to time, they are also fed thawed silversides for variety, and have also been fed live saltwater mollies.
Feeding can be done by hand – the adults swim right to the surface at feeding and will eagerly take live or thawed frozen shrimp out of from your fingers, sometimes squirting you in the face with water from their funnels in the process. A feeding stick (a piece of rigid tubing with a 7.5 cm (3 inch) lengths of 80 pound test fishing line glued to it) can also be used to make sure that individual animals are getting food. For enrichment, the cuttlefish get appropriately sized live crabs or live shrimp introduced quietly into the tank to allow the cuttles to stumble upon them and hunt them at their leisure.

The display tank
When the hatchlings were around twelve weeks old they were ready to be put on display. A tank of approximately 450 L (120 gallons US) with dimensions of 122 cm x 61 cm x 61 cm (48”x24”x24”) that shared a common sump with three other tanks containing fish, inverts and corals was prepared for the cuttles by adding a mix of substrates, river rock, live rock, four large Sarcophyton sp., nine Acanthophyllia deshayesiana, and three Protoreastor sp. sea stars for clean up of uneaten cuttle food. Two 175 Watt MH pendants containing 20,000K bulbs were added to support the needs of the corals. A rigid airline tube bubbles air near the surface, and produces aesthetically pleasing glitter lines. The total system volume is approximately 1,165 L (300 gallons US). Filtration includes various filter socks on the tank returns, a small fluidized reactor containing granular ferric oxide media and activated carbon, and an ASM G4+ protein skimmer. A remote deep sand bed for natural nitrate reduction is planned for the near future. As a result, water quality is maintained near the parameters described above.

Initially, thirty juvenile S. bandensis, approximately 2.5 cm (1 inch) in mantle length, were introduced to the display. Since the animals were so small, and so good at camouflage, the idea was to saturate the exhibit with cuttles to make it easier for guests to see them. A graphic of a small cuttle on the substrate was also added to give the guests an idea of what to look for. The plan was to remove animals from the display as they got bigger and began to show sexual characteristics and possible aggression. It seems cuttles can tell the sex of other cuttles on sight, but aquarists can only tell the difference through dominance postures (which aren’t always accurate) or by directly observing mating.
Over the next several months, that strategy worked out well. The S. bandensis ate and grew and the males began to show themselves by facing of with each other, stretching and widening their bodies while darkening their patterns in a presumed effort to assert sexual dominance. There were a few losses, noticeable by the discovery of cuttlebones with beak bites missing from them: looking like a surfboard after a shark attack. It is unclear if the losses were due to natural aggression or cannibalism resulting from inadequate quantities of food or frequency of feedings.
As the animals matured, some were removed from the display to holding tanks behind the scenes, leaving a population of six S. bandensis on display: four females, one large male and one smaller male. Since the males are generally the aggressors in this species, the larger/smaller relationship was settled on in order to curtail dominance fighting.

Mating
Like other cuttles, S. bandensis mate ‘fact to face’, intertwining arms for several minutes. Mating was observed in the animals on display at around sixteen weeks. Interestingly enough, while the larger male would be facing off with his reflection in the acrylic, the smaller male would be mating. As soon as the larger male noticed, the smaller male would stop, or be prevented from mating by the larger male.
Eggs
Even though mating had been witnessed for approximately four weeks, eggs weren’t discovered in the exhibit until the S. bandensis were about twenty weeks old. Eggs were laid one at a time, with a bit of ink incorporated into the ‘skin’ of the egg. Each egg took between two and five minutes to be laid and placed into the egg cluster, which is often attached to a rock in the exhibit. Clusters can be built up over several days and can range in size from a few eggs to 40 or 50 eggs. There does seem to be some post-laying parental interest in the eggs, with both the female and dominant male jetting water over the eggs from time to time for a few days after laying. It is unclear if clusters are laid by only one female at a time, or if several females can build clusters at the same time.

As the eggs developed and swelled they were moved off display and back into the critter keeper behind the scenes where we raised the parents. The eggs hatched in approximately one month.
Before working at the Steinhart Aquarium the author had bred S. bandensis several times in his home cephalopod breeding system. Although many eggs were laid the hatch rate was very low. In contrast, the amount of eggs laid at the Steinhart Aquarium was surprising, as was the number of eggs that hatched. Between August and November, approximately 600 eggs were laid on display, with the majority of them being viable. Over 300 eggs and hatchlings have been sent to other institutions. Egg laying didn’t end in December, but it did slow down noticeably. It will be interesting to see if fecundity drops off as the breeding group gets older. The author believes much of this breeding success is attributable to the amount of live food always available at the Steinhart Aquarium. The author’s home setup, didn’t allow for the housing of Cragnon spp., so there were frozen and fed out as needed. However, at the Aquarium frozen food was only kept as an emergency backup, and feed live food was fed twice a day instead. Further study is needed to determine the relationship between fresh and frozen food on fecundity of S. bandensis.
Preparing for the next group
With the success of the S. bandensis breeding on display, we replaced the critter keeper in back of house with a cube system plumbed into the same coral grow out system. There are now three cubes 30 cm x 25 cm x 25 cm (12”x10”x10”), fed by the same Maxijet 1200 power head, and gravity draining into the same sump that were used before. At the time of writing there are approximately thirty three-month-old cuttles, approximately eighty one-month-old cuttles along with several clutches of eggs both behind the scenes and on display. There are also several three-month-old S. bandensis that were purchased as eggs in September for genetic diversity when it appeared we would be successful with our breeding program.

Final thoughts
The refined group of S. bandensis on display has been remarkably stable with very little fighting over time.
The amount of water flow in the display is fast enough for the Sarcophyton to visibly sway back and forth. S. bandensis don’t seem to care if the flow is fast or slow, and don’t seem to be working very hard to stay in position in the areas of higher flow.
The display of S. bandensis has been very successful from a husbandry standpoint and a guest experience standpoint – the cuttles are very popular with both docents and guests. Feeding time is especially popular!
We look forward to breeding more of these animals and sharing both display and breeding stock with other institutions.
Acknowledgements
The author would like to thank:
Matt Wandell, J. Charles Delbeek, Seth Wolters, Nancy Levine, April Devitt and Pam Montbach for helping to feed and care for these animals. Bart Shepherd for approving and supporting the display and breeding program of dwarf cuttlefish. J. Charles Delbeek, Bart Shepherd, Chris Andrews and Laura Kormos for their input on this article. Chris Maupin and Dr. James Wood for their help when beginning to keep and breed S. bandensis, and all of the members of www.TONMO.com for their help and support over the years.
References
Ross, R. “Sepia bandensis; Husbandry and breeding.” Tropical Fish Hobbyist, August 2009: (pp102-106)
Internet References
Ross, R. “Keeping and breeding the dwarf cuttlefish Sepia bandensis.” Advanced Aquarist, September 2005: http://www.advancedaquarist.com/2005/9/aafeature. Republished on TheCephalopodPage May 2007: http://thecephalopodpage.org/Sepiabandensis.php.